Krypton Hexafluoride . An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. It was the first compound of krypton discovered. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. in this video we'll write the correct formula for krypton hexafluoride. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature.
from stock.adobe.com
heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. It was the first compound of krypton discovered. An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. in this video we'll write the correct formula for krypton hexafluoride. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers.
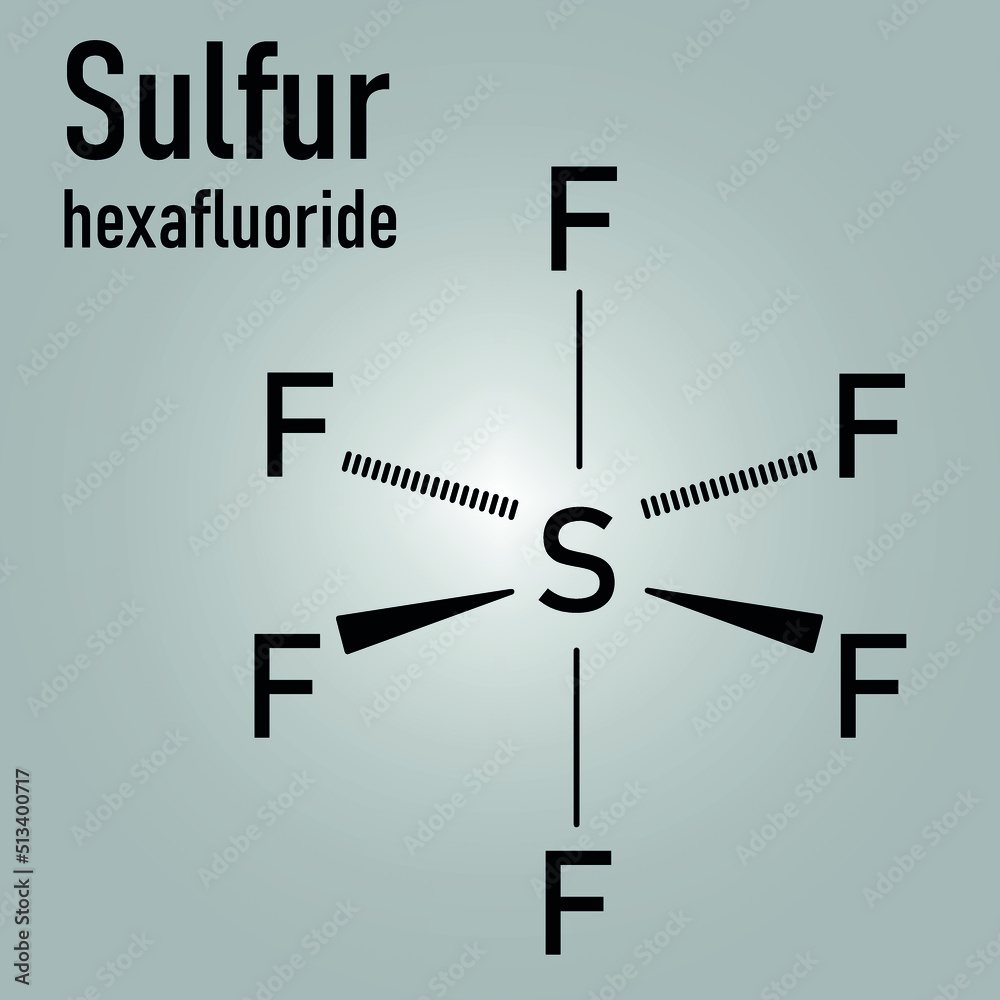
Skeletal formula of sulfur hexafluoride gas insulator molecule
Krypton Hexafluoride tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. It was the first compound of krypton discovered. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. in this video we'll write the correct formula for krypton hexafluoride. An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second.
From www.wikiwand.com
Krypton hexafluoride Wikiwand Krypton Hexafluoride It was the first compound of krypton discovered. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a. Krypton Hexafluoride.
From www.dreamstime.com
Krypton. Noble Gases. Chemical Element of Mendeleev S Periodic Table Krypton Hexafluoride heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. It was the first compound of krypton discovered. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. in. Krypton Hexafluoride.
From www.chegg.com
Solved 16. Given the equation Kr + 3F2 → KrF6, how many Krypton Hexafluoride in this video we'll write the correct formula for krypton hexafluoride. An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high. Krypton Hexafluoride.
From stock.adobe.com
Skeletal formula of sulfur hexafluoride gas insulator molecule Krypton Hexafluoride An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. It was the first compound of krypton discovered. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which. Krypton Hexafluoride.
From qd-ruiming.en.made-in-china.com
99.999 50L High Purity Carbon Dioxide Helium Krypton Xenon Neon Krypton Hexafluoride An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. tl;dr xenon hexafluoride has a fluxional structure in. Krypton Hexafluoride.
From www.dreamstime.com
F2Kr Krypton Difluoride CAS 13773814 Chemical Substance in White Krypton Hexafluoride Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. krypton difluoride, krf 2 is a chemical compound of. Krypton Hexafluoride.
From www.dreamstime.com
Krypton chemical symbol stock illustration. Illustration of square Krypton Hexafluoride It was the first compound of krypton discovered. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. in this video we'll write. Krypton Hexafluoride.
From www.alamy.com
Krypton, Kr, periodic table element with name, symbol, atomic number Krypton Hexafluoride An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. in this video we'll write the correct formula for krypton hexafluoride. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal. Krypton Hexafluoride.
From www.youtube.com
How to Write the Formula for Krypton hexafluoride YouTube Krypton Hexafluoride tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. in this video we'll write the correct formula for. Krypton Hexafluoride.
From www.alamy.com
Krypton on the periodic table of the elements Stock Photo Alamy Krypton Hexafluoride An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. It was the first compound of krypton discovered. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. in this. Krypton Hexafluoride.
From pubs.acs.org
Mutual and Thermal Diffusivities in Binary Mixtures of nHexane or 1 Krypton Hexafluoride Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. It was the first compound of krypton discovered. krypton difluoride, krf. Krypton Hexafluoride.
From www.chegg.com
Solved 16. Given the equation Kr + 3F2 → KrF6, how many Krypton Hexafluoride in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. in this video we'll write the correct formula for krypton hexafluoride. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. Krypton forms a difluoride, krf 2 , which is thermally unstable at. Krypton Hexafluoride.
From www.researchgate.net
Figure S6. Pressure dependence of the resonance frequency of NCD Krypton Hexafluoride heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. An electric discharge through such a tube gives a very. Krypton Hexafluoride.
From www.chegg.com
Solved 16. Given the equation Kr + 3F2 → KrF6, how many Krypton Hexafluoride in this video we'll write the correct formula for krypton hexafluoride. tl;dr xenon hexafluoride has a fluxional structure in the gas phase, with multiple rapidly interconverting conformers. It was the first compound of krypton discovered. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. An electric discharge through such a tube gives a. Krypton Hexafluoride.
From pubs.acs.org
Solubility and Liquid Density of Binary Mixtures of nHexane or 1 Krypton Hexafluoride Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. in this video we'll write the correct formula for krypton hexafluoride. krypton difluoride, krf 2 is a chemical compound of krypton and fluorine. It was. Krypton Hexafluoride.
From www.dreamstime.com
Ball and Stick Model of Plutonium Hexafluoride Molecule Stock Image Krypton Hexafluoride Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. in this video we'll write the correct formula for krypton hexafluoride. in particular we focus on krypton tetrafluoride, krf4, a molecular crystal in which krypton forms short. It was the first compound of krypton discovered. tl;dr xenon hexafluoride has a fluxional structure in. Krypton Hexafluoride.
From www.researchgate.net
Examples of spectra obtained from laser−produced plasmas created by Krypton Hexafluoride An electric discharge through such a tube gives a very intense light that lasts only \(\dfrac{1}{50,000}\) of a second. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. It was the first compound of krypton discovered.. Krypton Hexafluoride.
From www.thoughtco.com
Noble Gas Chemical Compounds Krypton Hexafluoride Krypton forms a difluoride, krf 2 , which is thermally unstable at room temperature. It was the first compound of krypton discovered. heats of formation of krypton fluorides and stability predictions for krf4 and krf6 from high level electronic structure. in this video we'll write the correct formula for krypton hexafluoride. krypton difluoride, krf 2 is a. Krypton Hexafluoride.